Introduction
Performance enhancement in athletic disciplines often revolves around compounds that offer an edge in muscle building, recovery, or resilience. Among these, ACE-031 – a peptide engineered for muscle growth regulation – has surfaced as a subject of intrigue.
While the theoretical benefits have drawn considerable attention, the human evidence base remains comparatively young. This comprehensive, evidence-driven guide critically examines ACE-031’s potential applications, limitations, and safety profile for athletes, bodybuilders, and coaches seeking a reliable, practical understanding.
Key Takeaways
- ACE-031 is a peptide that acts as a myostatin inhibitor, theoretically encouraging muscle growth by removing a biological brake on muscle development.
- Human studies on ACE-031 are limited to rare disease populations; direct athlete data is lacking.
- Athletic, bodybuilding, and performance enhancement use is not approved, and long-term effects are largely unknown in healthy adults.
- Reported benefits are context-dependent and individual responses vary widely.
- Caution is warranted due to insufficient safety data and off-label usage risks.
Quick Facts Table
| Aspect | Detail |
|---|---|
| Compound Name | ACE-031 |
| Compound Type | Peptide (myostatin inhibitor) |
| Main Proposed Benefit | Increases muscle mass by blocking myostatin pathway |
| Human Athlete Evidence | Very limited; most studies in children with neuromuscular disorders |
| Common Usage Contexts | Research, investigational drug, off-label enhancement interest |
| Legality | Not approved for sports or bodybuilding; banned by WADA |
| Safety Concerns | Unknown long-term effects, bleeding, blood pressure alterations, possible vascular effects |
| Typical Delivery Method | Injectable (subcutaneous or intramuscular) |
| Status | Research compound, not available for legal over-the-counter sale |
| Regulatory Status | Investigational; not FDA/EMA approved for muscle enhancement |
What is ACE-031?
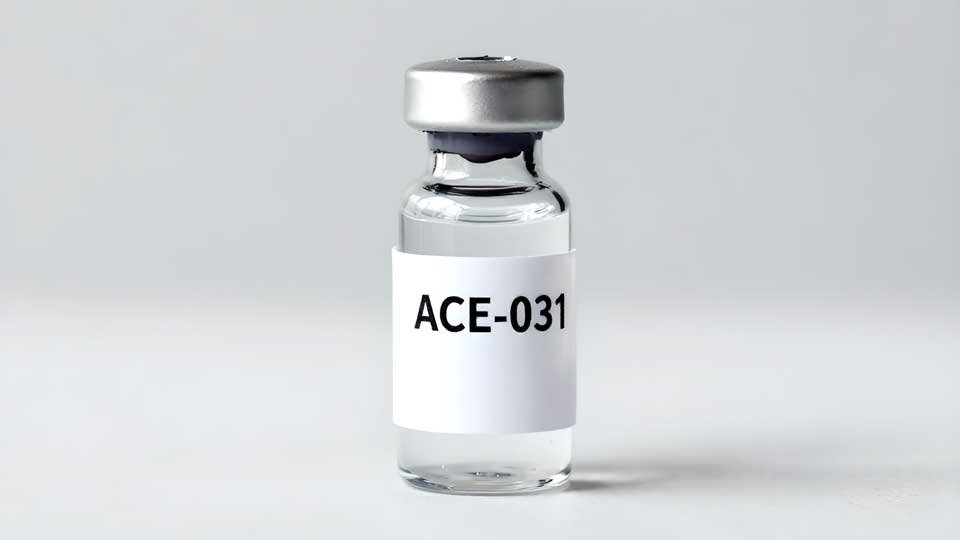
ACE-031 is a synthetic fusion peptide designed to disrupt the action of myostatin, a protein that typically limits muscle growth in humans. By blocking this protein, ACE-031 aims to remove a key restraint on muscle development, offering theoretical support for increased lean mass. Developed initially to address muscle-wasting diseases, it has garnered substantial interest among athletes and bodybuilders for its possible muscle-building effects.
History & Development
ACE-031 emerged from research into treatments for Duchenne muscular dystrophy and other conditions marked by severe muscle loss and degeneration. The compound was created through recombinant DNA technology, fusing part of the human activin receptor type IIB to an antibody fragment. Early preclinical studies demonstrated notable muscle growth in animals by inhibiting the myostatin pathway – a finding that catalyzed human trials. However, most published research centers on disease populations, not healthy, athletic individuals. Despite initial promise, safety concerns led to the discontinuation of several clinical programs, and development for broader uses stalled.
How ACE-031 Works
Mechanism of Action
ACE-031 acts as a decoy receptor for myostatin, essentially binding to the protein and neutralizing its activity. Myostatin, a member of the transforming growth factor-beta (TGF-β) family, normally signals muscles to limit their own growth. By intercepting this signal, ACE-031 reduces myostatin’s suppression of muscle development. This action theoretically allows for:
- Enhanced muscle protein synthesis
- Increased muscle fiber size
- Reduced muscle degradation
Biological Effects
In humans, inhibiting myostatin could theoretically yield increases in muscle mass and potentially improve muscle strength and function. Preliminary clinical studies outside the athletic population have explored whether these benefits translate into real-world functional improvements. However, broader biological consequences may arise, including effects on vascular health and other tissues sensitive to TGF-β family signals. The full spectrum of actions, especially with long-term or high-dose use, is not yet understood.
Benefits of ACE-031
Human research on ACE-031 has largely targeted populations with severe muscle wasting (such as children with muscular dystrophy), not healthy athletes or bodybuilders. As such, much of the theorized benefit for sports and bodybuilding rests on biological plausibility and limited disease cohort outcomes. The following summarizes possible benefits with an emphasis on the state of consensual human evidence.
Increased Lean Muscle Mass
Controlled trials indicate that ACE-031 can cause modest to moderate increases in muscle mass in pediatric disease populations. The effect in healthy, resistance-trained athletes is unknown. Results appear to depend on baseline muscle wasting and individual genetic factors.
Potential Improvements in Muscle Function
Data in humans suggest small improvements in functional tests (such as walking distance or stair climbing) in individuals with muscle-wasting disorders. Translating these results to well-trained athletes should be done with caution. Performance impacts may be less pronounced without underlying disease.
Muscle Preservation During Catabolic States
Evidence remains limited, but physiological rationale supports a possible role in reducing muscle loss during severe disuse, illness, or prolonged caloric deficits. No controlled trials have examined this use in athletes with high physical demands or during contest preparation.
Reduction in Myostatin Activity
Human studies confirm ACE-031 decreases circulating myostatin and related proteins. While this underpins the theorized muscle benefit, broader health consequences of chronic myostatin suppression remain undetermined in otherwise healthy people.
Improvements in Body Composition (Theoretical)
Some users report changes in body fat or lean/fat ratios, though data in athletes or healthy adults is lacking. Controlled evidence is limited to clinical populations and has not demonstrated dramatic physique alterations outside of disease states.
Potential Positive Effects on Bone Density (Experimental)
Early investigations hint that myostatin inhibitors could support bone health, but quality data in humans are sparse, and long-term outcomes are not established.

Side Effects & Safety
Most safety information comes from controlled trials in children with neuromuscular diseases. Some adverse effects have caused clinical trials to be halted, emphasizing the need for significant caution.
Bleeding and Nosebleeds (Epistaxis)
Human studies have reported an increased risk of bleeding, particularly nosebleeds, thought to be related to effects on small blood vessels and angiogenesis.
Changes in Blood Pressure
Subtle but statistically significant changes in blood pressure and blood vessel health have been observed. The long-term risk remains unclear, and some individuals may be more susceptible to cardiovascular side effects.
Interactions (if applicable)
Potential exists for interactions with drugs affecting the TGF-β pathway or vascular health. Because ACE-031 is not approved for medical use outside of research, there are no well-documented drug interaction profiles in athletes.

Pros & Cons
| Pros | Cons |
|---|---|
| Biological plausibility for muscle preservation | Lack of human sports/athlete data |
| Theoretically increases muscle mass | Serious potential side effects (bleeding, vascular) |
| Direct myostatin inhibition mechanism | Long-term safety unknown |
| Could decrease muscle loss in disease states | Legally and ethically restricted use |
| Rapid onset of biological effects | Requires injection; possible immune reactions |
| Interest in bone density support | Development for enhancement discontinued |
How Athletes & Bodybuilders Use ACE-031
Practical Usage Scenarios
There are no approved or recommended uses for ACE-031 in athletes or bodybuilders. All reported use is experimental and occurs outside sanctioned medical contexts. Scenarios reported or discussed among enhancement communities include:
- Attempting to break through plateaus in lean mass development
- Seeking anticatabolic effects during extreme dieting, injury, or high-volume training
- Exploration during post-injury rehabilitation phases (unapproved)
Dosing, Timing & Forms
Data on optimal dosing derives entirely from rare disease trials, which are not generalizable to healthy adults. No evidence-based protocols exist for enhancement, and deviation from research dosages may profoundly impact safety.
| Population | Dose Range (mg/kg or total) | Administration | Typical Frequency | Cycle/Duration | Notes |
|---|---|---|---|---|---|
| Pediatric (research) | 1–3 mg/kg | Subcutaneous | Every 2–4 weeks | 8–24 weeks (studied) | Disease setting only |
| Hypothetical Adult (none) | Unknown; not established | Subcutaneous | Unknown | Unknown | No evidence-based dose for adults |
| Male vs Female | No data on sex variance | — | — | — | — |
| With or Without Food | Not relevant (injectable) | — | — | — | — |
| Time of Day | Not documented | — | — | — | — |
| Bodyweight Consideration | Was weight-based in trials | — | — | — | Safe adult dose not known |
All unapproved use falls outside accepted medical safety protocols.
Monitoring & Safety Notes
Athletes considering or exposed to ACE-031 in any context should pursue clinical-grade supervision. Regular monitoring of blood counts, blood pressure, and signs of abnormal bleeding is critical. Spontaneous side effects may occur even at research doses.
Comparison to Similar Compounds
Overview
| Compound | Mechanism | Human Athlete Data | Main Application | Legal Status | Key Risks |
|---|---|---|---|---|---|
| ACE-031 | Myostatin inhibitor | No | Experimental muscle | Banned/Investigational | Bleeding, vascular, unknown |
| Myostatin Antibody | Myostatin Blockade | No | Muscle wasting | Banned/Investigational | Immune, unknown |
| SARMs | Androgen-AR binding | Limited | Lean mass | Banned (WADA) | Hormonal, cardiovascular |
| Anabolic Steroids | AR activation | Yes | Muscle, performance | Banned (WADA) | Multiple organ systems |
| GH (HGH) | Growth hormone | Yes (limited) | Recovery/mass | Prescription/controlled | Glucose, edema, joint issues |
Analysis
Most alternatives have more human safety data and a longer track record of use in athletic populations, albeit with substantial risks. ACE-031’s unique mechanism may offer an advantage in certain catabolic conditions but lacks both real-world athlete validation and a robust safety profile. Its off-label and research status sets it apart from substances with (albeit risky) clinical indications and established monitoring protocols.
Legality & Regulatory Status
ACE-031 is not approved for any consumer or over-the-counter use. All sports governing bodies—including the World Anti-Doping Agency (WADA)—explicitly ban its use. Possession, purchase, or sale of ACE-031 outside of authorized research is likely illegal in most jurisdictions. Regulatory authorities have halted or restricted clinical investigations due to safety concerns.
Where to buy “ACE-031”?
ACE-031 is not legally sold for non-research use. Any products marketed online as ACE-031 are either black-market, misbranded, or counterfeit – carrying significant legal and health risks. No reputable supplement, pharmacy, or sports nutrition retailer offers ACE-031. Athletes attempting to source the compound risk legal prosecution and significant harm from unknown or contaminated products.
Alternatives to ACE-031
Safer, legal alternatives for muscle support include adequate dietary protein, creatine supplementation, and evidence-backed resistance training protocols. Prescription medications for muscle wasting (such as anabolic steroids) are only used under close medical supervision for specific clinical indications and carry substantial risks. No natural supplement currently offers similar myostatin inhibition in humans.
Frequently Asked Questions (FAQ)
1. Is ACE-031 safe for healthy adults?
Current evidence does not support the safety of ACE-031 in healthy adults. Most research has stopped due to safety concerns even in sick populations.
2. Can ACE-031 help bodybuilders gain muscle?
There is no well-controlled human evidence showing reliable muscle gains in bodybuilders or trained athletes using ACE-031.
3. How is ACE-031 administered?
It is given as an injection, most often subcutaneously.
4. Are there natural ways to inhibit myostatin?
No dietary supplement or protocol offers reliable myostatin inhibition comparable to ACE-031 in human research.
5. What are the main health risks?
Increased bleeding, vascular issues, and unknown long-term effects are chief concerns.
6. Is ACE-031 legal for competitive athletes?
No. It is banned by all major anti-doping bodies and considered illegal for enhancement use.
7. Can women use ACE-031?
There is no safety or dosing data specific to women. No regulatory agency supports use in healthy females.
8. What if someone is caught using ACE-031 in sports?
Loss of eligibility, legal consequences, and potential health risks are possible.
9. How long does ACE-031 stay in the body?
Clearance rates are not well characterized in healthy adults. The biological effects may persist after dosing stops.
10. Is it possible to monitor for ACE-031 use?
Yes, anti-doping labs can detect ACE-031 and related markers in blood.
11. Does ACE-031 increase bone strength?
Preliminary findings suggest possible effects, but this is not proven in healthy humans.
12. Are there interactions with other PEDs or supplements?
No interaction data is available. Stacking unknown compounds increases risk.
13. Are counterfeit ACE-031 products dangerous?
Yes. Impurities, incorrect dosing, and untested compounds are common on the black market.
14. Can myostatin inhibitors cure muscle-wasting diseases?
They offer some promise but are not cures. Research has been limited by safety issues.
15. How does ACE-031 compare to SARMs or steroids?
All have serious risks, but at least SARMs and steroids have been studied more widely in humans. None are recommended for healthy enhancement.
Conclusion
ACE-031 remains a compound of scientific and athletic interest because of its myostatin-inhibiting properties. However, there is a significant lack of athlete or bodybuilder evidence, and serious safety concerns persist.
The compound is not legal for sports use, carries unknown long-term effects, and its benefits for healthy populations have not been demonstrated. Athletes and coaches should prioritize proven methods—rigorous training, adequate recovery, and established supplements—over experimental use of ACE-031.
Studies / References
- Studies in children with Duchenne muscular dystrophy evaluated subcutaneous ACE-031 (1–3 mg/kg every 2–4 weeks, up to ~24 weeks). Some muscle mass gains were recorded, but bleeding events and blood pressure changes led to trial suspension. Major limitation: population was severely ill, not healthy athletes; very short follow-up.
- A small pilot study using ACE-031 in pediatric neuromuscular disease looked at muscle function and bulk over ~3–6 months. Some improvement in functional scores but no major athletic performance measurement; effects did not clearly translate into daily life improvement. Limitation: disease-specific; rapid cessation due to safety events.
- Early-phase safety research in adults with cachexia or muscle loss after illness used single or repeat ACE-031 doses. Minimal functional benefit, some transient muscle mass increases, and adverse events (bleeding, hypertension) noted. Limitations include tiny sample size, short duration, and lack of athletic endpoints.
- Most subsequent clinical research was discontinued around efficacy and safety concerns, so information remains incomplete on applicability to healthy persons or performance environments.
Athlete Final Checklist
- Is the compound legal and ethically permitted for your competition? ❌
- Is there robust evidence supporting benefit in athletes or bodybuilders? ❌
- Is the safety profile supported by multiple long-term human studies? ❌
- Do you have access to qualified medical oversight? ❌
- Are proven, safe alternatives available? ✅
Pursue only evidence-backed, legal pathways for performance enhancement. Stay vigilant for evolving science, but prioritize your health and career integrity above speculative interventions like ACE-031.